Visceral artery aneurysms (VAAs) are a quite rare but potentially life-threatening vascular disorder involving predominantly the splenic and the common hepatic arteries and less frequently the coeliac, left gastric or branches of the superior mesenteric arteries. The incidence is between 1% and 2% in the general population and splenic artery aneurisms represent approximately two-thirds of them.1–4 VAAs are usually detected and treated when they reach a threshold diameter of 2 cm. Very rarely they may silently grow and reach a size larger than 5 cm. In such cases, they are referred to as ‘giant’ VAAs (GVAAs).5–7 GVAAs may be discovered as an incidental finding on a scan, or they may cause compression symptoms – mainly to the stomach – or present as a rupture.8–10 Because of the risk of rupture, surgical or endovascular treatment is required imminently when such lesions are discovered.
Management of Giant Visceral Artery Aneurysms
Surgical repair has historically been the treatment of choice for all visceral aneurysms.11,12 However, a less invasive endovascular approach has also been gradually adopted in a number of centres – in line with local expertise and available tools – and is now the first option in most centres.13–21 Both surgical and endovascular approaches present challenges in the treatment of these lesions and these are described in more detail in the following sections.
Surgery
Most commonly, GVAAs originate from the splenic artery. Giant aneurysms originating from the hepatic artery or the coeliac trunk are much less common and carry a higher element of complexity when surgical repair is attempted.6,22 For giant aneurysms of the splenic artery, aneurysmectomy with or without splenectomy is expected to be more feasible for lesions located in the proximal or the distal third of the vessel.23,24 For giant splenic artery aneurysms located in the mid portion of the artery, therefore attached to the pancreas, simple ligation of the proximal and distal segments of the artery might be the preferred strategy. Aneurysm ligation without vascular reconstruction is also the preferred option in acute settings for haemodynamically unstable patients. For hepatic GVAAs, aneurysmectomy with vessel reconstruction is required for lesions that are located distally to the origin of the gastroduodenal artery whereas simple ligation may be performed for proximal lesions because of the presence of the collateral circuit.25 Coeliac giant aneurysms are extremely rare and most of them are diagnosed in post mortem examinations – in part because these lesions can also be infectious.26 Surgical approaches also include aneurysmorrhaphy and revascularisation with vein or prosthetic grafts. If the aneurysm includes trifurcation, complexity is significantly increased and mortality reaches 5%.27
Surgical access might be obtained with standard laparotomy – mainly for splenic giant aneurysms.28 This would comprise a left subcostal approach, dissection of the greater omentum with ligatures up to the omental sac followed by dissection and clamping of the two ends of the splenic artery and aneurysmectomy. For coeliac lesions, access might be more challenging. The coeliac trunk may not be accessible anteriorly because of the large size of the mass and a thoracoabdominal incision with retroperitoneal access to the vessels through the gastrocolic ligament might be required.24 Another similar approach might be performed with a chevron incision and rotation of the viscera medially.25 Sometimes control of the artery may be difficult to achieve and additional manoeuvres might be necessary such as medial rotation of the upper abdominal viscera to gain retroperitoneal access to the abdominal aorta. Laparoscopic approaches may also be used as an alternative given the lower morbidity, although there is very limited experience for the treatment of GVAAs.29,30
In cases where arterial reconstruction that would preserve flow towards the organs distally to the aneurysm is not possible, additional visceral resection may be required.31 Surgical options include splenectomy or even distal pancreatectomy when necessary.32,33 Multi-step procedures and combined treatment (both endovascular and surgical) may also be considered in complicated cases and successful use of this approach in the management of a patient with multiple GVAAs has been reported.34
Although data on the surgical management of GVAAs are limited, it is reasonable to assume that morbidity and mortality would increase proportionally for larger lesions. On the other hand – given the dramatic improvement in endovascular techniques and materials – endovascular approaches may also be feasible for challenging cases with unfavourable anatomy and complex sac morphology and are now considered as the first treatment option.
Endovascular Management
The endovascular approach is associated with early postoperative recovery and consequently shorter hospital stays. It represents a valid alternative in high-risk patients with multiple comorbidities and those with a history of abdominal surgery for whom intraperitoneal adhesions may be a concern.
An essential prerequisite in planning endovascular treatment is the availability of good quality, high-resolution imaging. This should include dynamic CT scans and multi-planar reconstructions in order to assess vascular anatomy of the district and determine the most appropriate treatment strategy.
When dealing with GVAAs, endovascular management may be more challenging and many operators are concerned about potential complications and long-term outcomes. Moreover, scientific literature is lacking in studies concerning the management of GVAAs and only a few case reports have been described.35–39
Spiliopoulos et al. advocated the efficacy of endovascular treatment in larger size aneurysms.40 They reported a high clinical and technical success rate dealing with VAAs with a mean diameter of 49.4 ± 21 mm and visceral artery pseudoaneurysms (VAPAs) with a mean diameter of 25.1 ± 14.6 mm. Procedural technical success was achieved in all cases. The target lesion re-intervention rate was 6.1% (2/33 cases) in the VAPA group and 14.2% (3/21) in the VAA group.
A recent systematic review by Hamid et al. analysed 92 cases of giant splenic artery aneurysm.41 Endovascular treatment was considered successful in 89.7% of patients (35/39). The researchers compared endovascular and surgical treatments and noted a comparable efficiency in the reduction of aneurysm-related death and palliation of aneurysm-related symptoms. The study also revealed a higher rate of post-procedure complications with endovascular treatment (p<0.05). However, this outcome may be subject to further interpretation. In fact, in the scenario of splenic artery GVAAs, embolisation of the feeding vessels is often strictly related to the possible onset of splenic infarcts and it can be considered as a minimum loss compared with the risk of splenectomy. So, the authors agreed that endovascular therapeutic techniques – even in the scenario of GVAAs – may be associated with a lower rate of major complications.41
Considerations for Endovascular Techniques
Endovascular management of GVAAs is challenging and requires a combination of several techniques and materials from the interventional armamentarium, including coils, vascular plugs, liquid embolic agents and covered stents. Moreover, the procedure can be performed in a multistage fashion, in order to occlude the several efferent vessels progressively and reduce radiation exposure to the patient.42 The strategy may represent a risk due to the dynamic flow and pressure change inside the sac that may lead to a sudden rupture.
Length of the neck, tortuosity of the arteries, the precise location of the aneurysm and angulation of the aneurysmal tract should be evaluated carefully prior to deciding the embolisation device and technique. It should also be taken into account that a combination of several treatment techniques might be necessary in most cases.
The double inflow-outflow blockage technique is of paramount importance in order to diminish the risk of reperfusion. All the efferent vessels that originate from the sac – usually multiple in giant aneurysms – need to be embolised in order to obtain a complete exclusion of lesion. The outflow vessels should be addressed first.
Selective micro-catheterisation of small arteries originating from huge sacs is a rather challenging task and, in some cases, partial embolisation of the aneurysmal sac helps in reducing the flow and visualising all the feeding vessels. In these cases, the use of liquid embolic agents is very useful as they may reach distal vessels.35
The endovascular coiling approach has been demonstrated to be effective even in very large aneurysms, and more than sufficiently fast and safe to use in the event of a ruptured visceral aneurysm and a sudden drop in blood pressure.36
The packing technique, to prevent the risk of endoleaks, due to the large space in the sac, may be performed using different embolic agents such as fragments of guidewire, long detachable coils (only recently available for peripheral interventions) or liquid agents in order to obtain a better thrombosis of the aneurysm. However, isolated sac packing has been reported to be associated with a high risk of coil compaction and recanalisation of the aneurysmatic sac.19 In fact, in partially thrombosed aneurysms, even sufficient packing does not preclude recanalisation of the aneurysm because factors other than compaction, such as thrombus resolution and migration of coils into the thrombus, cause the aneurysm to recanalise over time. Therefore, in giant-sized aneurysms, the presence or not of sac thrombus may be of paramount importance, as sufficient coil packing to prevent revascularisation would result in significant additional cost, time, and radiation exposure to the patient with equivocal overall benefit.35,36,42–44 In order to achieve a better post-coiling result, the use of the combination of liquid embolics, such as ethylene vinyl alcohol copolymer (Onyx), with coil has been reported with good results.37
The use of covered stents, although virtually appealing for preserving the vessel patency as reported in the literature, is not feasible in most cases for dealing with GVAAs because of the absence of an optimal landing zone, significant tortuosity and evidence of an infected sac.19,38
Even the use of some interesting techniques derived from the endovascular neurointervention experience has been applied in the case of GVAAs. For example, Gjoreski et al. reported a case of GVAA of the hepatic artery successfully treated with dual-layer stents placement as a flow-diverting option.39
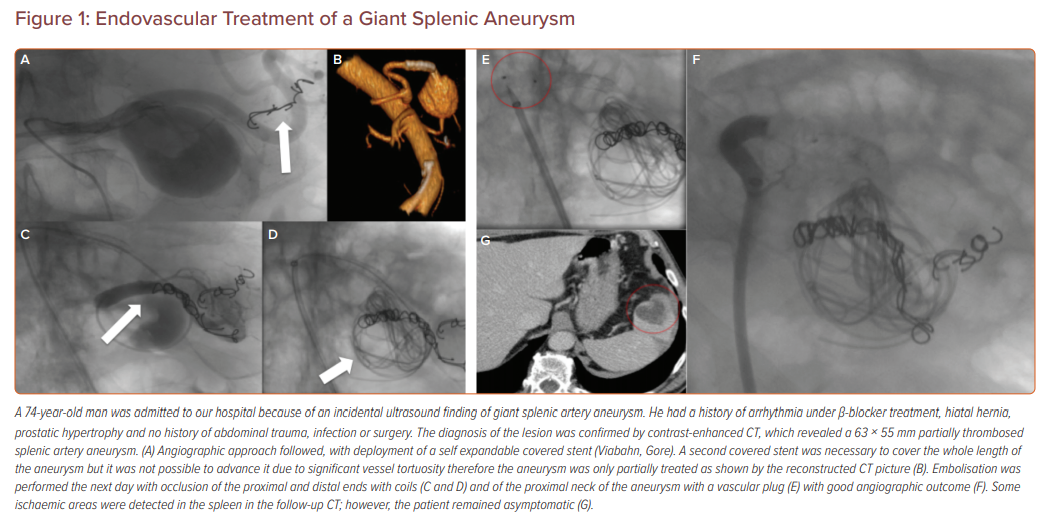
Figure 1 describes the management of a patient in our hospital after the incidental ultrasound discovery of a giant splenic artery aneurysm.
Conclusion
In summary, the management of GVAAs is a complex issue that has to be carried out in very experienced centres. Preliminary studies available in the literature suggest that endovascular treatment is efficient and safe and may be considered as the first-line approach thanks to the low associated morbidity and mortality. However, when considering endovascular exclusion for GVAAs of the abdominal cavity, it is recommended that the patient is haemodynamically stable, there are no signs of aneurysm rupture, there is an experienced team of interventional radiologists and the surgical team is aware and on-board in case of complications or acute rupture. Further studies are necessary to assess the efficacy of this technique in the treatment of haemodynamically unstable patients due to the rupture of the sac.